The activation of atmospheric CO2 and its chemical fixation as metal carbonate complexes are of continuous attention for the purpose of fundamental research but also in terms of potential application to reduce the concentration of this greenhouse gas. Carbon dioxide is slightly soluble water. In aqueous medium gaseous CO2 is in equilibrium with carbonic acid (H2CO3) through the following reactions:
CO2(g) ⇌ CO2(aq) CO2(aq) + H2O(l) ⇌ H2CO3(aq)
The equilibrium constant for the reaction at 25°C is Kh = 1.70×10−3. This means that most of the carbon dioxide remains as CO2 molecules, rather than being converted into carbonic acid.
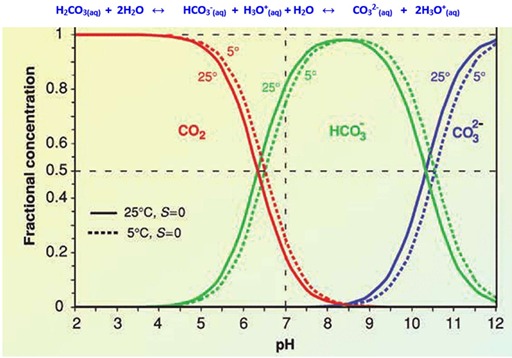
It is well known that at pH ~ 6.0 carbonic acid is in equilibrium with bicarbonate anion and at pH ~ 10 bicarbonate anion is in equilibrium with carbonate anion (Scheme 1). This equilibrium suggests that in alkaline medium, solubility of CO2 increases and majorly exists as carbonate anion (CO32-).
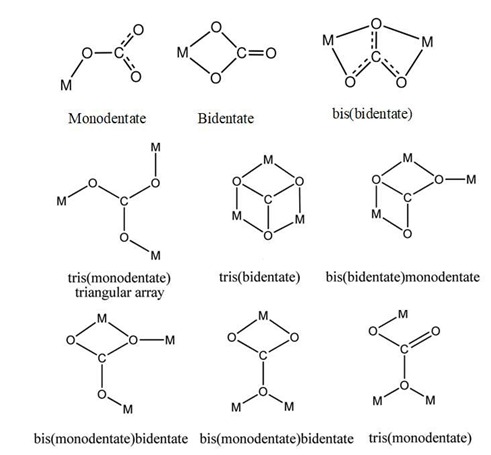
This carbonate anion can act as monodentate and bidentate ligands binding a single metal ion or as a bridging ligand linking more than one metal ions through its various coordination modes (Scheme 2).
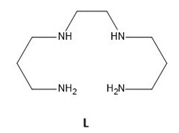
In this present endeavor, we have explored the coordination behavior of carbonate anion in alkaline medium for the spontaneous chemical fixation of atmospheric CO2 in the form of crystalline zinc(II) amine carbonate complex. In aqueous medium, we have taken zinc(II) perchlorate hexahydrate salt and a tailored tetradentate amine, N,N’-bis(3-aminopropyl)-1,2- ethanediamine (L, Scheme 3) in 1:1 molar ratio in a beaker and left undisturbed in an open air.
After 2-3 days colourless crystalline compounds appeared at the surface of the solution as well at the bottom of the beaker. This compound is then collected by filtration and dried in air-oven at 105 °C. The formation of zinc(II) amine carbonate complex has been established through FTIR spectrum of the isolated compound. In FTIR spectrum, ν(N–H) stretching frequencies of the –NH2 groups of L is observed at ~3230 cm-1. Several weak bands in the range 2880–2950 cm-1 assignable to aliphatic C–H stretching vibration are observed. The asymmetric stretching vibrations of carbonate are seen at 1480 and 1440 cm-1. The presence of perchlorate stretches at 1150, 1120, 1080 and 620 cm-1 are indicative of non-coordination to the metal centre. The formulation of the isolated complex as [Zn3(μ3-CO3)(L)3](ClO4)4 (1) is established by single crystal X-ray diffraction analysis (Scheme 4). Structural analyses show that the complex 1 consist of one trinuclear [Zn3(μ3-CO3)(L)3]4+ cation and four perchlorates as counter anions. The formation of carbonate complex 1 may presumably be due to intake of CO2 from atmosphere by the alkaline zinc(II) amine species that leads to transformation of CO2 into CO 2- anion at pH ~11 in aqueous solution followed by coordinating three metal centres [Zn(L)]2+ through tris(monodentate) bridging mode (Scheme 4).
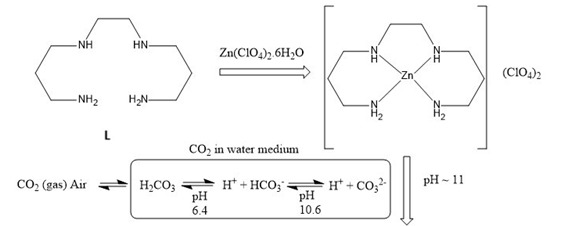
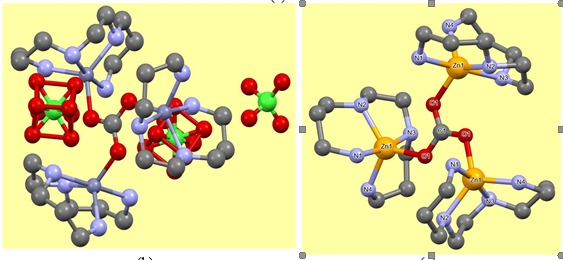
Scheme 4 (a) Formation of carbonate complex and molecular structure of (b) [Zn3(μ3- CO3)(L)3](ClO4)4 (1) and (c) [Zn3(μ3-CO3)(L)3]4+ cation
This present work illustrates that Zinc (II) ion in combination with judiciously chosen polyamine creates suitable alkaline medium which triggers the fixation of atmospheric carbon dioxide into carbonate complex. We are now active to investigate such fixation of this greenhouse gas to some other metal ions such as Copper (II), Nickel(II), Cobalt(II) and Cadmium(II) in combination with L and other polydentate amines.
Contributed by Dr. Kishalay Bhar


Chief Operation, FAMD, Tata Steel Limited..


Sr. General Manager,, Emirates Trading Agency L.L.C..


Mines Manager, Hindustan Zinc Limited, a Vedanta Company.


General Manager, Stevin Rock L.L.C..


Executive Vice President (Works),, DCW Limited.


AVP – Coal Quality & Sales Compliance Head,, PT Indo Tambangraya Megah Tbk (BANPU).


Laboratory Head, MMX.


Shipping Administrator, Mount Gibson Iron Limited.


Senior Director – Asia Pacific Iron Ore Sales,, Cliffs Natural Resources Pty Ltd..


Member, Compass Group (India) Pvt. Ltd.

Posted on December 16 2025 By Mitra S.K ADMIN
Read More
Posted on December 16 2025 By Mitra S.K ADMIN
Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 11 2025 By Mitra S.K ADMIN
![Estimating Cobalt by UV-Vis Spectroscopy: The [CoCl?]²? Acetone Method](https://mitrask.com/uploads/blogs/1764834098Estimating%20Cobalt.png)
Posted on December 04 2025 By Mitra S.K ADMIN
Posted on December 04 2025 By Mitra S.K ADMIN

Posted on November 12 2025 By Mitra S.K ADMIN

Posted on September 23 2025 By Mitra S.K ADMIN

Posted on August 01 2025 By Mitra S.K ADMIN

Posted on July 25 2025 By Mitra S.K ADMIN

Posted on July 18 2025 By Mitra S.K ADMIN

Posted on July 01 2025 By Mitra S.K ADMIN

Posted on May 22 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 03 2024 By Mitra S.K ADMIN

Posted on October 17 2024 By Mitra S.K ADMIN

Posted on October 04 2024 By Mitra S.K ADMIN

Posted on September 13 2024 By Mitra S.K ADMIN

Posted on August 27 2024 By Mitra S.K ADMIN

Posted on August 23 2024 By Mitra S.K ADMIN

Posted on June 27 2024 By Mitra S.K ADMIN

Posted on June 22 2024 By Mitra S.K ADMIN

Posted on June 15 2024 By Mitra S.K ADMIN

Posted on May 24 2024 By Mitra S.K ADMIN
Posted on May 17 2024 By Mitra S.K ADMIN

Posted on May 09 2024 By Mitra S.K ADMIN

Posted on April 20 2024 By Mitra S.K ADMIN

Posted on April 13 2024 By Mitra S.K ADMIN

Posted on April 30 2024 By Mitra S.K ADMIN

Posted on April 29 2024 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 26 2023 By Mitra S.K ADMIN

Posted on April 05 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on June 14 2022 By Mitra S.K ADMIN






