
Introduction:
Silica (SiO2) is a highly abundant mineral found in the earth's crust. It comprises several minerals and is the principal component of over 95% rocks. Silica naturally occurs in both amorphous and crystalline forms. In crystalline silica, silicon atoms are bonded to four oxygen atoms forming tetrahedrons which are connected to each other in a three dimensional array, different from a random pattern in amorphous silica. Additionally, crystalline silica appears in several polymorphic forms, namely quartz, cristobalite and tridymite, with quartz being the most common. The transformation of crystalline silica into its different forms occurs with variation of temperature and pressure. At atmospheric pressure, pure quartz can remain stable at up to 870° C and can transform into tridymite above 870° C, and tridymite can further transform into cristobalite at temperature higher than 1470° C (American J. of Science 1913). However, several studies reported that when quartz is heated, it converts directly to cristobalite at about 1050 ° C, while tridymite is produced only in the presence of mineralizing agents such as alkali oxides. Few other studies have found that cristobalite is first produced from quartz and then cristobalite transforms into tridymite (Environmental Pollution 2022).
Respirable crystalline silica, very small particles with diameter less than 10 µM, can be found in beaches and playgrounds and is created during cutting, sawing, grinding, drilling and crushing stone, rock, concrete, brick and mortar. Exposure to these tiny particles of silica at elevated levels can lead to health effects such as silicosis, lung cancer and kidney disease. The toxicity of silica is highly dependent on its size and crystal form. The order of fibrogenic effect is found as follows: tridymite > cristobalite> quartz > amorphous silica.
Apart from the traditional occupational exposures, coal and biomass burning can provide high temperature environments that can change the crystal forms of silica, leading to risk for respirable silica exposure. Therefore, considering the possible changes in the crystal structure of silica during burning processes and the toxicities of different forms of silica, it is important to understand the crystal transformation pathways during coal and biomass burning for the assessment of its exposure risks. Herein, we have investigated the crystal transformation pathways of silica during the coal and biomass burning processes and clarified in detail the influence of coexisting elements in the crystal transformations of silica.
Materials and Methods
For our combustion experiments, four types of coal samples were collected, two of which are of Indian origin (Coal A and B) and two from Indonesia (Coal C and D). Three types of biomass samples were collected including bamboo stick, biomass pellet and rice husk. High purity silica sample (BCS 313/2) was used as quartz standard in the burning experiments.
The burning experiments of coal and biomass samples were performed by combustion at different temperatures in a muffle furnace. Samples were put into the muffle furnace in a porcelain crucible and kept at a specific temperature for 5 hours when it reached the highest temperature.
Powder XRD analysis was carried out for phase characterization of coal and biomass ashes obtained after combustion at different temperatures. Ash composition of the samples was determined by XRF analysis.
Results and Discussion
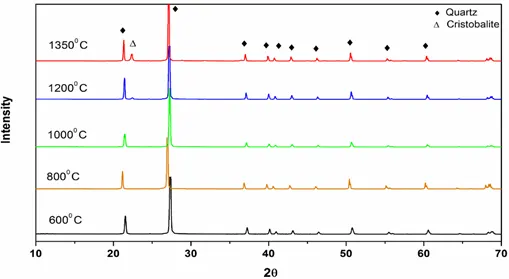
Figure 1: XRD patterns of high purity quartz after burning at different temperatures.
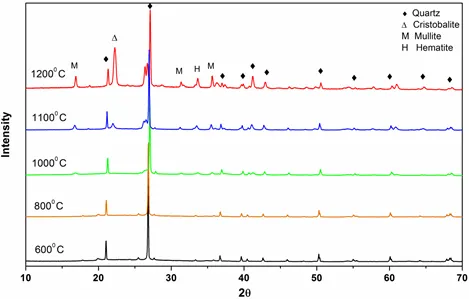
Figure 2: XRD patterns of ashes of Coal A obtained at different temperatures.
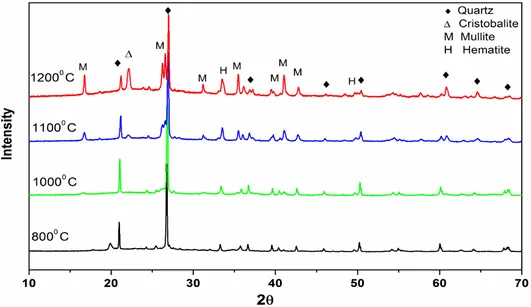
Figure 3: XRD patterns of ashes of Coal B obtained at different temperatures.
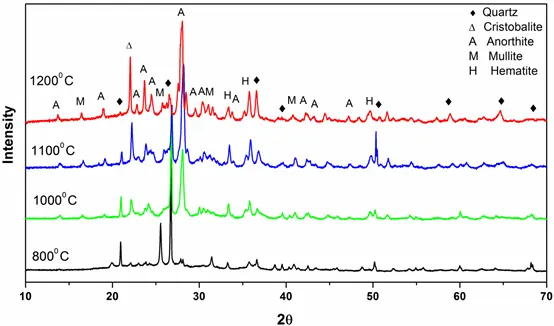
Figure 4: XRD patterns of ashes of Coal C obtained at different temperatures.
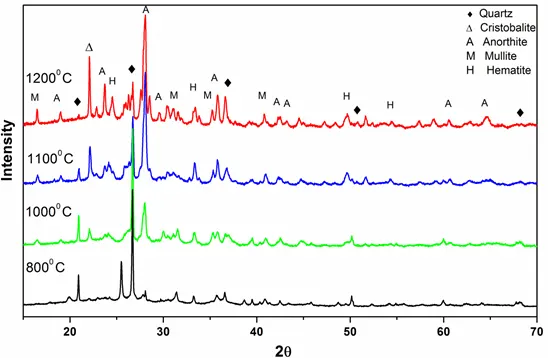
Figure 5: XRD patterns of ashes of Coal D obtained at different temperatures
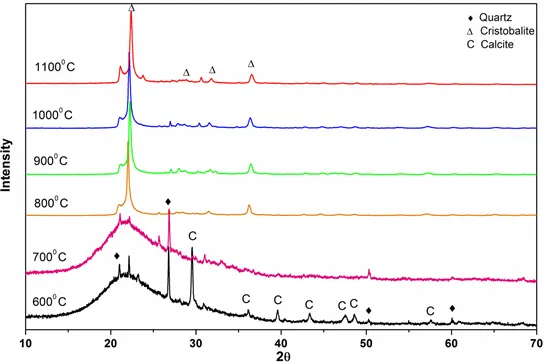
Figure 6: XRD patterns of bamboo ashes obtained at different temperatures.
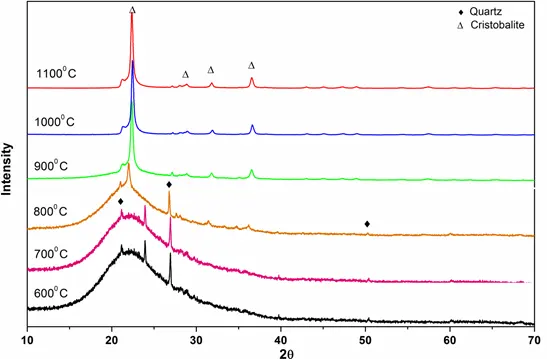
Figure 7: XRD pattern of biomass pellet ashes obtained at different temperatures.
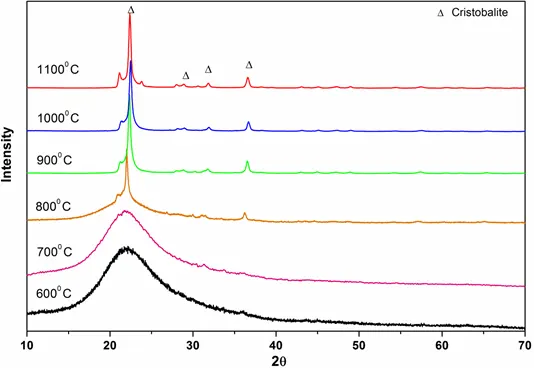
Figure 8: XRD patterns of rice husk ashes prepared at different temperatures
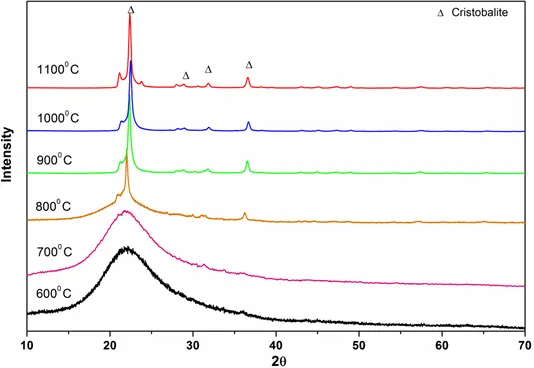
Figure 9: XRD patterns of ashes of Coal B blended with rice husk
XRD patterns of pure quartz after combustion at different temperatures (600° C- 1350° C) are shown in Fig. 1. From the characteristic diffraction peaks, we found that pure quartz remains stable up to 1200° C and it began to transform into cristobalite above 1200 ° C. Figs 2 and 3 show that for coals, quartz started to transform into cristobalite at a temperature of 1100° C. Again, for Coal C and D as shown in Fig 4 and 5, cristobalite formation began at a temperature of 1000° C.
For biomass samples, as indicated by Figs 6 to 8, the transformation to cristobalite started at 800° C. The XRD patterns of biomass samples from 600° C to 800° C, show curved surfaces, probably due to the amorphous nature of silica. We know that biogenic silica or phytoliths are widely present in plants and the major component of this phytoliths is the amorphous silica. Hence, for biomass burning, the amorphous silica could directly transform into cristobalite starting at 800° C.
Under coal burning environments, we found that cristobalite formation started at a temperature (1000° C) lower than that for pure quartz (>1200° C). It is therefore possible that some substances in coal catalyze the crystal transformation of silica during burning processes. Alkali metal ions (e.g., Na, K, Fe and Al) are known to be able to catalyze the nucleation of cristobalite on the quartz grains and lower the crystal transformation temperature. From Table 1, we can see that all of the coals contain a high amount of alkali oxides (Na2O, K2O, Fe2O3 and Al2O3), which would assist the crystal transformation of quartz into cristobalite.
Table 1: Ash compositions of coals and biomasses
|
Minerals |
Coal A |
Coal B |
Coal C |
Coal D |
Biomass (Bamboo) |
Biomass (Pellet) |
Rice Husk |
|
SiO2 |
63.19 |
59.87 |
51.07 |
51.41 |
86.7 |
94.72 |
94 |
|
Al2O3 |
19.98 |
23.62 |
25.63 |
24.82 |
0.76 |
0.68 |
0.09 |
|
Fe2O3 |
3.72 |
6.67 |
5.63 |
5.78 |
0.91 |
0.52 |
0.46 |
|
CaO |
0.03 |
0.52 |
5.57 |
4.69 |
4.5 |
0.73 |
0.5 |
|
MgO |
0.2 |
0.99 |
1.64 |
1.57 |
0.84 |
0.55 |
0.33 |
|
P2O5 |
0.18 |
0.42 |
0.45 |
0.49 |
1.05 |
0.87 |
0.5 |
|
MnO2 |
0.02 |
0.12 |
0.21 |
0.13 |
0.19 |
0.19 |
|
|
TiO2 |
1.79 |
1.57 |
0.72 |
0.72 |
0.03 |
0.05 |
|
|
Na2O |
0.04 |
0.36 |
2.67 |
2.49 |
0.26 |
0.05 |
0.09 |
|
K2O |
0.83 |
1.7 |
1.09 |
1.27 |
3.81 |
1.38 |
1.66 |
Furthermore, in case of biomass burning, amorphous silica directly transforms into cristobalite from 800° C, which is much lower than the temperature obtained for coal burning. Smaller cations such as Li, Na and K have been shown to produce the crystalline silica polymorphs even when added in modest quantities (J. of European Ceramic Society, 2015). In particular, K significantly enhances the formation of cristobalite at a temperature of 800° C by stabilizing its crystal structure. Since, all of the biomass ashes exhibit a high abundance of K which further increases the catalytic efficiency by facilitating the formation of cristobalite and thereby resulting in the lower critical transformation temperature (800° C).
Another interesting finding in our study is that when coal B is blended with 20% rice husk, the transformation of quartz into cristobalite from 1100° C to 1200° C was greatly enhanced as can be seen from the intensity of cristobalite peak in Fig 9. Here, the intensity of the cristobalite peak obtained for blended coal is much higher than that obtained for combustion of coal B alone in Fig 3.
Conclusion
Coal and biomass burning processes are considered as common sources for respirable silica exposure. Here we found that in coal burning process, quartz transforms into cristobalite from 1000° C, while in case of biomass burning, amorphous silica transforms into cristobalite from 800° C. In general, coal burning generates a high temperature environment, suggesting a high probability for the wide occurrence of crystalline silica in various places where coal is burned. Biomass burning poses even more risks as it produces crystalline silica at a much lower temperature. Nowadays, thermal power plants use biomass in combination with coal which can increase the health risk of exposure to combustion related particles.
Contributed by Dr. Sudeb Ghosh


Chief Operation, FAMD, Tata Steel Limited..


Sr. General Manager,, Emirates Trading Agency L.L.C..


Mines Manager, Hindustan Zinc Limited, a Vedanta Company.


General Manager, Stevin Rock L.L.C..


Executive Vice President (Works),, DCW Limited.


AVP – Coal Quality & Sales Compliance Head,, PT Indo Tambangraya Megah Tbk (BANPU).


Laboratory Head, MMX.


Shipping Administrator, Mount Gibson Iron Limited.


Senior Director – Asia Pacific Iron Ore Sales,, Cliffs Natural Resources Pty Ltd..


Member, Compass Group (India) Pvt. Ltd.

Posted on January 16 2026 By Mitra S.K ADMIN
Read More
Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN
Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 11 2025 By Mitra S.K ADMIN
![Estimating Cobalt by UV-Vis Spectroscopy: The [CoCl?]²? Acetone Method](https://mitrask.com/uploads/blogs/1764834098Estimating%20Cobalt.png)
Posted on December 04 2025 By Mitra S.K ADMIN
Posted on December 04 2025 By Mitra S.K ADMIN

Posted on November 12 2025 By Mitra S.K ADMIN

Posted on September 23 2025 By Mitra S.K ADMIN

Posted on August 01 2025 By Mitra S.K ADMIN

Posted on July 25 2025 By Mitra S.K ADMIN

Posted on July 18 2025 By Mitra S.K ADMIN

Posted on July 01 2025 By Mitra S.K ADMIN

Posted on May 22 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 03 2024 By Mitra S.K ADMIN

Posted on October 17 2024 By Mitra S.K ADMIN

Posted on October 04 2024 By Mitra S.K ADMIN

Posted on September 13 2024 By Mitra S.K ADMIN

Posted on August 27 2024 By Mitra S.K ADMIN

Posted on August 23 2024 By Mitra S.K ADMIN

Posted on June 27 2024 By Mitra S.K ADMIN

Posted on June 22 2024 By Mitra S.K ADMIN

Posted on June 15 2024 By Mitra S.K ADMIN

Posted on May 24 2024 By Mitra S.K ADMIN
Posted on May 17 2024 By Mitra S.K ADMIN

Posted on May 09 2024 By Mitra S.K ADMIN

Posted on April 20 2024 By Mitra S.K ADMIN

Posted on April 13 2024 By Mitra S.K ADMIN

Posted on April 30 2024 By Mitra S.K ADMIN

Posted on April 29 2024 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 26 2023 By Mitra S.K ADMIN

Posted on April 05 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on June 14 2022 By Mitra S.K ADMIN






