DRI is a premium ore-based metallic raw material made by removing chemically-bound oxygen from iron oxide pellets and lump ores without melting. Direct reduction refers to solid-state processes which reduce iron oxides to metallic iron at temperatures below the melting point of iron. The reducing agents can be carbon monoxide and hydrogen, coming from reformed natural gas, syngas or coal. The DRI resembles a honeycomb structure, which looks spongy in texture when viewed in microscope and hence is also called ‘sponge iron’.
Use of DRI provides an alternative way of steel production as the production involves much lesser temperature compared to the Blast furnace. Consequently, understanding of the DRI composition is very important for the steel making purpose.
Direct reduction processes can be divided roughly into two categories: gas-based (75% production) and coal-based (25% production). In both cases, the objective of the process is to remove the oxygen contained in various forms of iron ore (sized ore, concentrates, pellets, mill scale, furnace dust) in order to convert the ore to metallic iron, without melting it (below 1200 °C). The following reactions successively convert hematite (from iron ore) into magnetite, magnetite into ferrous oxide, and ferrous oxide into metallic iron by reduction with CO/H2 at a high temperature of 800 - 1200 °C:
3Fe2O3 + CO/H2 → 2Fe3O4 + CO2 /H2O Fe3O4 + CO/H2 → 3 FeO + CO2 /H2O FeO + CO/H2 → Fe + CO2 /H2O
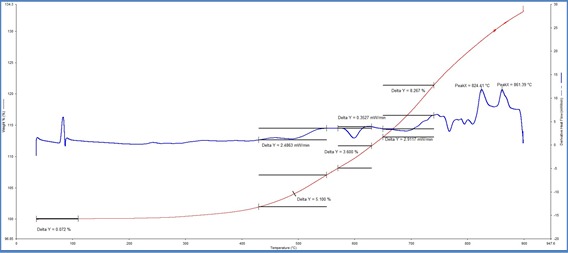
TG curve for one DRI sample
To understand the chemistry of the DRI, two industrial samples were analyzed using various techniques including thermogravimetry and powder X ray Diffraction. Conventional wet analysis and ICP OES were also employed to understand the elemental composition. The elemental composition is summarized in table 1.
|
% COMPOSITION |
DRI Sample 1 |
DRI Sample 2 |
METHODOLOGY |
|
Total Iron (FeT) |
83.71 |
83.55 |
Reduction of dissolved sample to ferrous state; titration with standard dichromate solution |
|
Metallic Iron (FeM) |
80.22 |
80.64 |
Addition of neutral mercuric chloride solution to dry sample; titration with standard dichromate solution |
|
Silica (SiO2) |
7.63 |
7.45 |
Volatilization of silica residue with HF |
|
Carbon (C) |
0.20 |
0.19 |
C-S Analyzer |
|
Sulphur (S) |
0.02 |
0.02 |
|
|
Calcium Oxide (CaO) |
0.80 |
0.78 |
Trace element analysis by ICP-OES |
|
Magnesium Oxide (MgO) |
0.22 |
0.20 |
|
|
Aluminium Oxide (Al2O3) |
6.46 |
6.58 |
|
|
Titanium Oxide (TiO2) |
0.27 |
0.23 |
|
|
Nickel Oxide (NiO) |
0.11 |
0.07 |
|
|
Phosphorus (P) |
0.03 |
0.03 |
For thermogravimetric analysis (TGA), samples were first run in nitrogen atmosphere till
105℃ and then in zero air (N2:O2 ~ 80:20) up to 950℃. A loss in weight percentage at 105℃ gives us the moisture content in the samples. Three humps were observed in the TGA curves which correspond to three major phase changes:
(i) Fe→FeO at 450-550℃
(ii) FeO→Fe3O4 at 570-630℃ (iii) Fe3O4→ Fe2O3 at 650-760℃
X-ray diffraction of the powder samples shows a very sharp peak at around 2θ = 45° which corresponds to metallic iron content. A few smaller peaks were observed which may refer to a spinel structure bearing Al and Mg thereby confirming the presence of Al estimated in the wet analysis. Presence of relatively high Al indicates the use of bentonite instead of limestone during production.
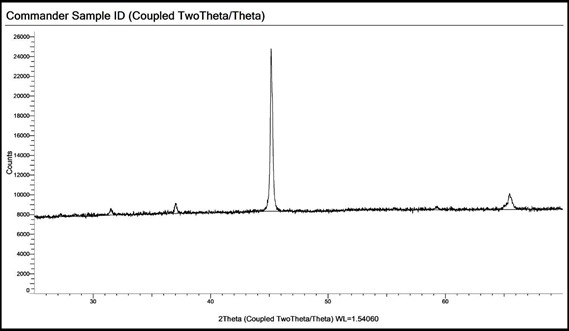
PXRD pattern of DRI sample 1
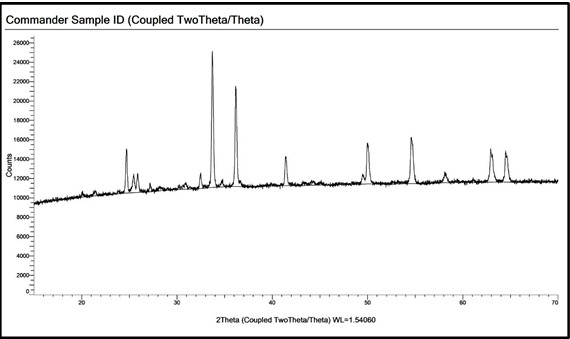
PXRD Pattern of the DRI sample 1 after ignition at 950°C
Furthermore, the DRI samples were ignited at 950℃ for one hour in the muffle furnace. The gain in weight before and after ignition was calculated. An XRD scan was performed on the ignited samples and the pattern resembles that of a typical hematite. We may conclude that the metallic iron in DRI has been oxidized to hematite at a temperature of 950℃.
|
|
DRI Sample 1 |
DRI Sample 2 |
|
% Weight Gain at 950℃ |
35.52 |
36.02 |
USES OF DRI :
Sponge iron is not useful by itself, but can be processed to create wrought iron or steel. India is the second-largest producer of steel on a global scale and DRI sector contributes significantly to the overall production of steel in India. Its physical and chemical characteristics make it desirable for use in the electric arc furnace (EAF), blast furnace (BF), and basic oxygen furnace (BOF). It is used to make a broad range of steel products including exposed auto body, extra deep drawing quality, fine wire, special bar quality, forging bar quality, plate and seamless tubes. DRI is most commonly made into steel using electric arc furnaces to take advantage of the heat produced by the DRI product. As evident, DRI is a useful raw material for steel production where use of very costly Blast furnace process can be skipped and therefore understanding of the DRI chemistry is essential. In this present work a pair of DRI samples were subjected to chemical analysis as well as Xray diffraction and thermogravimetric analysis. Stepwise weight gains were observed in the TG analysis corroborates to various reactions involved for the metal to different metal oxides at temperature ranges and the Xray Diffraction pattern confirms the presence of metallic iron as the major phase in the samples.
Contributed By: Asis Acharjee & Olivia Datta


Chief Operation, FAMD, Tata Steel Limited..


Sr. General Manager,, Emirates Trading Agency L.L.C..


Mines Manager, Hindustan Zinc Limited, a Vedanta Company.


General Manager, Stevin Rock L.L.C..


Executive Vice President (Works),, DCW Limited.


AVP – Coal Quality & Sales Compliance Head,, PT Indo Tambangraya Megah Tbk (BANPU).


Laboratory Head, MMX.


Shipping Administrator, Mount Gibson Iron Limited.


Senior Director – Asia Pacific Iron Ore Sales,, Cliffs Natural Resources Pty Ltd..


Member, Compass Group (India) Pvt. Ltd.

Posted on January 16 2026 By Mitra S.K ADMIN
Read More
Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN
Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 11 2025 By Mitra S.K ADMIN
![Estimating Cobalt by UV-Vis Spectroscopy: The [CoCl?]²? Acetone Method](https://mitrask.com/uploads/blogs/1764834098Estimating%20Cobalt.png)
Posted on December 04 2025 By Mitra S.K ADMIN
Posted on December 04 2025 By Mitra S.K ADMIN

Posted on November 12 2025 By Mitra S.K ADMIN

Posted on September 23 2025 By Mitra S.K ADMIN

Posted on August 01 2025 By Mitra S.K ADMIN

Posted on July 25 2025 By Mitra S.K ADMIN

Posted on July 18 2025 By Mitra S.K ADMIN

Posted on July 01 2025 By Mitra S.K ADMIN

Posted on May 22 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 03 2024 By Mitra S.K ADMIN

Posted on October 17 2024 By Mitra S.K ADMIN

Posted on October 04 2024 By Mitra S.K ADMIN

Posted on September 13 2024 By Mitra S.K ADMIN

Posted on August 27 2024 By Mitra S.K ADMIN

Posted on August 23 2024 By Mitra S.K ADMIN

Posted on June 27 2024 By Mitra S.K ADMIN

Posted on June 22 2024 By Mitra S.K ADMIN

Posted on June 15 2024 By Mitra S.K ADMIN

Posted on May 24 2024 By Mitra S.K ADMIN
Posted on May 17 2024 By Mitra S.K ADMIN

Posted on May 09 2024 By Mitra S.K ADMIN

Posted on April 20 2024 By Mitra S.K ADMIN

Posted on April 13 2024 By Mitra S.K ADMIN

Posted on April 30 2024 By Mitra S.K ADMIN

Posted on April 29 2024 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 26 2023 By Mitra S.K ADMIN

Posted on April 05 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on June 14 2022 By Mitra S.K ADMIN






