
An investigation to understand the efficacy of Xray Diffraction for the estimation of various Aluminum bearing phases in Bauxite in comparison to Thermogravimetric analysis:
Bauxite is the major source of Aluminum. Trihydrate alumina (Al2O3, 3 H2O) or Gibbsite, also known as low temperature alumina due to its lower extraction temperature is the major aluminum bearing material. Along with Gibbsite, Boehmite or Diaspore both of which are monohydrate alumina (Al2O3, H2O) are the other aluminum bearing materials in Bauxite. Extraction of these monohydrate phases require considerably higher temperature that leads to additional energy cost and thus accurate estimation of trihydrate and monohydrate alumina in Bauxite is of high importance from the viewpoint of economic viability.
The typical wet chemical approach to estimate trihydrate alumina involves caustic digestion in the range of 140°C to 150°C temperature whereas the estimation of the monohydrate alumina requires significantly higher temperature in excess of 200°C in presence of strong caustic solution. The process is extremely hazardous as it involves handling of caustic solution at elevated temperatures and can cause serious injuries to the persons involved. It also involves use of typical vessels with high durability and strict maintenance protocol of the experimental set up, thereby requiring a safer and faster alternative that can bypass use of hazardous chemicals.
In our laboratory we have studied 4 such bauxite samples using thermogravimetry and powder X ray diffraction to understand the mineralogical nature of the bauxite and subsequent quantification of Aluminum bearing phases were also attempted. The samples were subjected to TG analysis as well as Xray diffraction. Total Al2O3 content was also estimated using typical wet chemical approach that involves complexometric titration.
The TG curves were interpretated and from the weight loss patterns in the range of 150°C to
350°C and 450°C to 550°C respectively, estimation of try hydrate and mono hydrate alumina content were attempted. The summary of the weight loss and corresponding calculated aluminum bearing phase contents are tabulated in table 1.
|
Sample ID |
Weight Loss |
Corresponding trihydrate Alumina |
Weight loss |
Corresponding Monohydrate Alumina |
Total Al2O3 content |
|
1 |
16.386 |
47.35 |
2.370 |
15.81 |
44.22 |
|
2 |
15.172 |
43.84 |
2.653 |
17.70 |
31.43 |
|
3 |
16.386 |
47.35 |
2.370 |
15.81 |
43.54 |
|
4 |
12.257 |
35.42 |
2.620 |
17.48 |
37.881 |
On the other side, PXRD analysis were carried out for the same set of samples and phase quantification was attempted using Rietveld refinement. The phase composition of all the samples are tabulated in table 2
|
Sample ID |
Aluminium bearing Phases |
% Phase composition |
Total Al2O3 content |
% Al2O3 from wet analysis |
|
1 |
Diaspore (Al2O3, H2O) |
6.22 |
36.97 |
38.01 |
|
|
Boehmite (Al2O3, H2O) |
10.23 |
|
|
|
|
Gibbsite (Al2O3, 3 H2O) |
61.93 |
|
|
|
|
Kaolinite (Al2O3.2SiO2.2H2O) |
3.28 |
|
|
|
2 |
Diaspore (Al2O3, H2O) |
9.06 |
32.24 |
32.68 |
|
|
Boehmite (Al2O3, H2O) |
6.95 |
|
|
|
|
Gibbsite (Al2O3, 3 H2O) |
51.85 |
|
|
|
|
Kaolinite (Al2O3.2SiO2.2H2O) |
5.02 |
|
|
|
3 |
Diaspore (Al2O3, H2O) |
13.23 |
33.43 |
33.32 |
|
|
Boehmite (Al2O3, H2O) |
6.40 |
|
|
|
|
Gibbsite (Al2O3, 3 H2O) |
52.48 |
|
|
|
|
Kaolinite (Al2O3.2SiO2.2H2O) |
5.57 |
|
|
|
4 |
Diaspore (Al2O3, H2O) |
8.34 |
30.35 |
31.41 |
|
|
Boehmite (Al2O3, H2O) |
3.63 |
|
|
|
|
Gibbsite (Al2O3, 3 H2O) |
47.68 |
|
|
|
|
Kaolinite (Al2O3.2SiO2.2H2O) |
7.63 |
|
|
The total Alumina content of the samples were also measured with a classical wet technique and a comparison was made to understand the efficiency of the instrumental processes involved. The total alumina content obtained from the wet experiments was is in good agreement with the calculated total Al2O3 content obtained from the XRD experiments. On the other hand, the data obtained from the weight loss observed in the TG patterns resulted in much higher recoveries with comparison to the PXRD and wet analysis. This is consistent with the nature of the Gibbsitic material and a plethora of literature data can be found where a thermogravimetric factor is required to properly estimate the tri and monohydrate alumina contents using TG method which is beyond the scope of this work. From the powder Xray data, besides mono and trihydrate alumina phases, presence of kaolinite clay was also confirmed. The same is difficult to identify in the TG thermogram as no water loss peak was observed for kaolinite. The TG thermogram accounts for only the mono and trihydrate alumina and missed out on the clay mineral which will also contribute in the total Al2O3 obtained in the wet methods. As evident from the data, the PXRD analysis appears to be more robust as it does not require usage of certain factors which are purely empirical in nature and may often mislead. The PXRD method also employs a much larger sample quantity compared to the TG method and thus nullifies the issue of heterogeneity to a large extent. It can also provide information on all the crystalline aluminum bearing phases that is often not possible in the TG method.
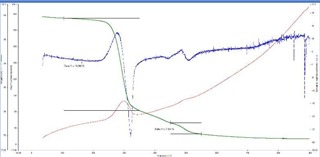
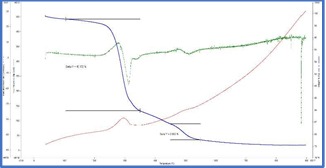
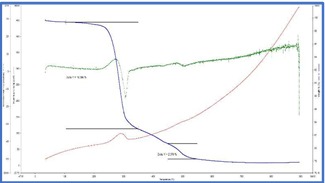
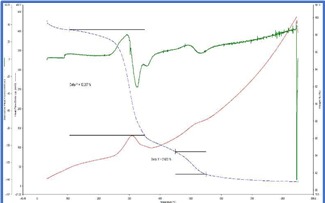
TG thermograms of samples 1-4 (Top to bottom)
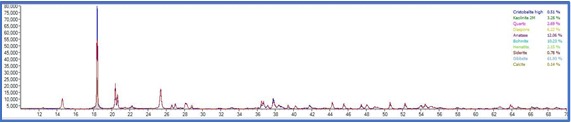
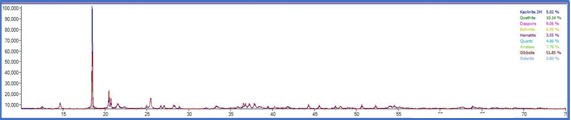
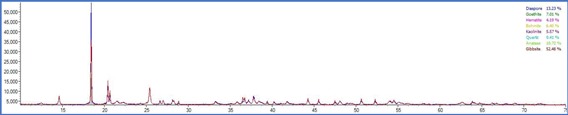
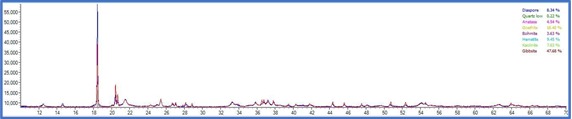
Although, for the purpose of the aluminum extraction the information of total trihydrate and monohydrate alumina content is sufficient and PXRD analysis is capable enough to provide information on that thereby providing an alternative analytical pathway which is rapid, effective, sufficiently accurate and does not involve hazardous chemicals and intensive energy consumption.
Contributed By: Dr. Arijit Goswami, Dr. Satirtha Sengupta and Alakta Saha


Chief Operation, FAMD, Tata Steel Limited..


Sr. General Manager,, Emirates Trading Agency L.L.C..


Mines Manager, Hindustan Zinc Limited, a Vedanta Company.


General Manager, Stevin Rock L.L.C..


Executive Vice President (Works),, DCW Limited.


AVP – Coal Quality & Sales Compliance Head,, PT Indo Tambangraya Megah Tbk (BANPU).


Laboratory Head, MMX.


Shipping Administrator, Mount Gibson Iron Limited.


Senior Director – Asia Pacific Iron Ore Sales,, Cliffs Natural Resources Pty Ltd..


Member, Compass Group (India) Pvt. Ltd.

Posted on February 19 2026 By Mitra S.K ADMIN
Read More
Posted on February 19 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN
Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 11 2025 By Mitra S.K ADMIN
![Estimating Cobalt by UV-Vis Spectroscopy: The [CoCl?]²? Acetone Method](https://mitrask.com/uploads/blogs/1764834098Estimating%20Cobalt.png)
Posted on December 04 2025 By Mitra S.K ADMIN
Posted on December 04 2025 By Mitra S.K ADMIN

Posted on November 12 2025 By Mitra S.K ADMIN

Posted on September 23 2025 By Mitra S.K ADMIN

Posted on August 01 2025 By Mitra S.K ADMIN

Posted on July 25 2025 By Mitra S.K ADMIN

Posted on July 18 2025 By Mitra S.K ADMIN

Posted on July 01 2025 By Mitra S.K ADMIN

Posted on May 22 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 03 2024 By Mitra S.K ADMIN

Posted on October 17 2024 By Mitra S.K ADMIN

Posted on October 04 2024 By Mitra S.K ADMIN

Posted on September 13 2024 By Mitra S.K ADMIN

Posted on August 27 2024 By Mitra S.K ADMIN

Posted on August 23 2024 By Mitra S.K ADMIN

Posted on June 27 2024 By Mitra S.K ADMIN

Posted on June 22 2024 By Mitra S.K ADMIN

Posted on June 15 2024 By Mitra S.K ADMIN

Posted on May 24 2024 By Mitra S.K ADMIN
Posted on May 17 2024 By Mitra S.K ADMIN

Posted on May 09 2024 By Mitra S.K ADMIN

Posted on April 20 2024 By Mitra S.K ADMIN

Posted on April 13 2024 By Mitra S.K ADMIN

Posted on April 30 2024 By Mitra S.K ADMIN

Posted on April 29 2024 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 26 2023 By Mitra S.K ADMIN

Posted on April 05 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on June 14 2022 By Mitra S.K ADMIN






