
A boiler is an enclosed vessel, which is used to transfer the heat generated from combustion of a fuel into water to convert it into steam. The steam so formed is used to drive certain industrial processes such as electricity generation. Boilers utilize coal, oil, natural gas or biomass as fuels for heat generation and they are designed in such a way to utilize maximum heat from the combustion reaction. Performance of a boiler mainly depends on heat transfer efficiency. Metals being good heat conductors, all the contact surfaces between the fire side and water side are either metal or metal alloys. During continuous operation of a boiler, scale formation on the exposed metal surfaces are unavoidable. The scaling on boiler tubes is predominantly governed by the feed water chemistry, which in turn affects the heat transfer efficiency of boilers. Deposition of scales and sludges on boiler tube walls leads to the following detrimental effects:
Continuously evaporating circulating water of the boiler leaves behind progressively increased concentrations of dissolved salts. Concentration of dissolved salts after reaching a saturation point tend to precipitate on the inner walls of the boiler. Loose and slimy precipitate, generally found on the colder parts of the boiler is called Sludge (e.g. CaCl2, MgCO3) while hard and adhering precipitates are called Scales (e.g. CaSiO3, Mg(OH)2).
Scales can be formed by various mechanisms from the components present therein. Ca(HCO3)2 decomposition can lead to the formation of a sticky CaCO3 scale in the following way:
Ca(HCO3)2 ⟶ CaCO3 ↓ + H2O + CO2 ↑
Similarly, scale formation can also occur by hydrolysis of Magnesium salts:
MgCl2 + 2 H2O ⟶ Mg(OH)2 + 2HCl
There are many reasons for scale formation like poor pre-treatment of the incoming water or contamination of the condensate which gets collected in the cooler parts of the boiler, where the circulating steam condenses.
Boiler corrosion which is detrimental for boiler health and subsequent performance can occur via various mechanisms. Pre-boiler corrosions occur when metals (like Iron or Copper) transport to boiler from external equipment. The corrosion can be due to various reasons. It can be either oxygen related corrosion, where metal surfaces start acting like galvanic cells, resulting in gradual corrosion of the surface or pH related corrosion, where the changing pH of the feed water can lead to degradation of the metal surface. The force of the water hitting against the different bends of the boiler tubes can lead to erosion of boiler surfaces, leading to corrosion. Excluding the external factors, internal boiler corrosion is a general concept that defines all corrosion mechanisms that originate inside the boiler tubes. Caustic corrosion, acid corrosion and Hydrogen embrittlement are the three main contributing factors of internal corrosion.
In a nutshell, different types of scales or deposits found at the various parts of boilers essentially demand identification/understanding of the chemistry comprising of a host of metals and metal bearing mineralogical phases. Consequently, a proper understanding of the scale/deposit composition is the key to the root cause analysis of the corrosion or performance issues that can considerably improve the health and safety aspects of the high value machinery and impact the economical side of the affairs in a positive note.
Experiments were carried out on 6 boiler sludge samples, which were received from a coal fired boiler (having a fire-side model). The composition of the sludges was of particular interest to us. This could be a helpful tool in analysing the working condition of the boilers and their efficiency. The techniques used for studying the sludges were:
The samples were sized down to 200 mesh and dried at 250C. They were then diluted with KBr and pelletized prior to subjecting them to IR spectroscopy on a Thermo Scientific Nicolet iS10 IR
spectrometer. The recorded IR spectra of the samples have been shown below (Fig.1)
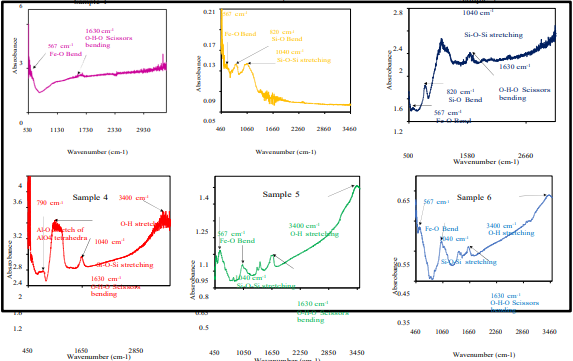
Fig. 1: IR Spectra of the boiler sludge samples
The IR spectra of the samples clearly indicate the presence of different phases of silica and Iron oxides in them. Other than these two major phases, aluminium oxides are also present. The IR peaks of O-H indicate the presence of moisture, which is rather unavoidable in spite of drying the samples at high temperature prior to measurement.
The samples were analysed in an ARLTM QUANT’X EDXRF Spectrometer. Table 1 represents the summarized elemental composition.
Table 1: Elemental composition of Sample 1-6
|
Sample 1 |
Sample 2 |
Sample 3 |
|||
|
Parameters |
% (m/m) |
Parameters |
% (m/m) |
Parameters |
% (m/m) |
|
Fe2O3 |
90.80 |
Fe2O3 |
34.80 |
Fe2O3 |
1.90 |
|
SiO2 |
3.45 |
SiO2 |
31.73 |
SiO2 |
96.99 |
|
Al2O3 |
2.46 |
CaO |
17.12 |
SO3 |
0.48 |
|
MnO |
1.61 |
Al2O3 |
7.12 |
Al2O3 |
0.25 |
|
Cr2O3 |
0.35 |
TiO2 |
2.15 |
Cr2O3 |
0.07 |
|
CaO |
0.34 |
MnO |
2.10 |
P2O5 |
0.13 |
|
ZnO |
0.26 |
MgO |
1.59 |
K2O |
0.07 |
|
TiO2 |
0.24 |
SO3 |
1.23 |
CuO |
0.03 |
|
NiO |
0.13 |
K2O |
1.15 |
MoO3 |
0.02 |
|
K2O |
0.11 |
MoO3 |
0.43 |
Cl |
0.02 |
|
Cl |
0.06 |
Cr2O3 |
0.20 |
MnO |
0.02 |
|
P2O5 |
0.07 |
P2O5 |
0.11 |
|
|
|
MoO3 |
0.04 |
CuO |
0.06 |
|
|
|
CuO |
0.03 |
ZnO |
0.05 |
|
|
|
|
|
|
|
|
|
|
Sample 4 |
Sample 5 |
Sample 6 |
|||
|
Parameters |
% (m/m) |
Parameters |
% (m/m) |
Parameters |
% (m/m) |
|
SiO2 |
66.02 |
Fe2O3 |
81.34 |
Fe2O3 |
21.17 |
|
Al2O3 |
21.46 |
SiO2 |
2.98 |
SiO2 |
4.06 |
|
CaO |
6.51 |
MnO |
0.89 |
CaO |
0.96 |
|
Fe2O3 |
2.04 |
Al2O3 |
0.81 |
SO3 |
0.44 |
|
K2O |
1.37 |
TiO2 |
0.80 |
MgO |
0.33 |
|
MgO |
1.31 |
Cr2O3 |
0.77 |
MnO |
0.19 |
|
ZnO |
0.34 |
SO3 |
0.41 |
K2O |
0.11 |
|
MnO |
0.33 |
BaO |
0.38 |
P2O5 |
0.08 |
|
|
|
K2O |
0.09 |
TiO2 |
0.05 |
|
|
|
MoO3 |
0.08 |
ZnO |
0.05 |
|
|
|
CuO |
0.08 |
Cl |
0.04 |
|
|
|
MgO |
0.07 |
CuO |
0.03 |
|
|
|
ZnO |
0.05 |
Cr2O3 |
0.02 |
The XRF results corroborates with the FTIR results and indicate presence of major phases of Silica and
Iron oxide, along with some other minor phases.
PXRD studies:
The samples (-200 mesh size) were subjected to X ray diffraction in a Bruker D2 Phaser powder X ray diffractometer. The PXRD patterns have been provided in Figure 2 and the composition of the samples have been provided in Table 2.
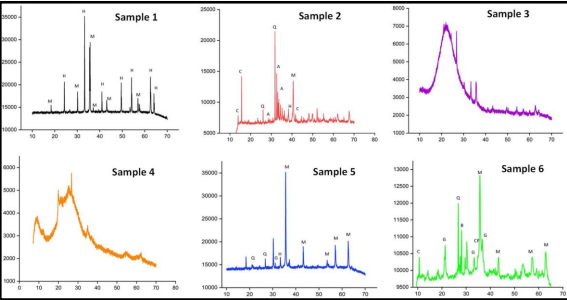
Fig. 2 PXRD patterns of the samples 1-6
Table 2 : Phase composition of samples 1-6
|
Sample 1 |
Sample 2 |
Sample 3 |
|||
|
Phase |
Chemical formula |
Phase |
Chemical formula |
Phase |
Chemical formula |
|
Hematite (H) |
Fe2O3 |
Coquimbite (C) |
AlFe3(SO4)6(H2O)12, 6H2O |
Amorphous |
NA |
|
Magnetite (M) |
Fe3O4 |
Hematite (H) |
Fe2O3 |
|
|
|
|
|
Magnetite (M) |
Fe3O4 |
|
|
|
|
|
Quartz (Q) |
SiO2 |
|
|
|
|
|
Anhydrite (A) |
CaSO4 |
|
|
|
Sample 4 |
Sample 5 |
Sample 6 |
|||
|
Phase |
Chemical formula |
Phase |
Chemical formula |
Phase |
Chemical formula |
|
Amorphous |
NA |
Quartz (Q) |
SiO2 |
Clinoptilolite (C) |
Na, Al, K, Ca silicate |
|
|
|
Goethite (G) |
FeO(OH) |
Boehmite (B) |
AlO(OH) |
|
|
|
Hematite (H) |
Fe2O3 |
Quartz (Q) |
SiO2 |
|
|
|
Magnetite (M) |
Fe3O4 |
Goethite (G) |
FeO(OH) |
|
|
|
|
|
Cuprite (CP) |
Cu2O |
|
|
|
|
|
Magnetite (M) |
Fe3O4
|
The XRD data of the sludge samples clearly reveals the presence of various phases, mainly comprising Iron oxides (Magnetite and Hematite), Silicon Oxides (SiO2). These data go hand in hand with the IR and XRF data, proving that the sludge and scales are mainly composed of Iron and Silicon Oxides. The Iron oxides might result from the corrosion of the steel tubes, while the feed water might be the source of the Silicon oxides.
Conclusion:
Here 6 sludge samples collected from a coal fired boiler were subjected to chemical analysis by employing FTIR, EDXRF and XRD technique. This revealed that the sludge samples mainly consist of Silicon and Iron oxides, resulting from contaminated feed water and corroded boiler tubes, respectively. As suggested by the current data set, the deposits mainly consist of Iron oxides and can be eliminated by proper deaeration of the feed water using suitable oxygen scavengers. Such corrosion of boiler tubes can also be prevented by pH monitoring of the incoming feed water. Although presence of considerable amount of Calcium and Magnesium salts are not observed in these samples, passing the feed water through a simple cation exchanging water softener is an answer to such issues. High Silica based sludges can be dealt with by proper filtration of the feed water. Thus, proper identification of the deposit composition is can provide the remedy and it remains the aim of our future work which will include thorough collection of sludge and scale samples from a variety of boiler models and predict their origin thereby finding the means to extend lifetime and enhance the performance of high value installations.
Contributed by Aradhita Bhattacharjee and Arijit Goswami


Chief Operation, FAMD, Tata Steel Limited..


Sr. General Manager,, Emirates Trading Agency L.L.C..


Mines Manager, Hindustan Zinc Limited, a Vedanta Company.


General Manager, Stevin Rock L.L.C..


Executive Vice President (Works),, DCW Limited.


AVP – Coal Quality & Sales Compliance Head,, PT Indo Tambangraya Megah Tbk (BANPU).


Laboratory Head, MMX.


Shipping Administrator, Mount Gibson Iron Limited.


Senior Director – Asia Pacific Iron Ore Sales,, Cliffs Natural Resources Pty Ltd..


Member, Compass Group (India) Pvt. Ltd.

Posted on February 19 2026 By Mitra S.K ADMIN
Read More
Posted on February 19 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on January 16 2026 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 16 2025 By Mitra S.K ADMIN
Posted on December 16 2025 By Mitra S.K ADMIN

Posted on December 11 2025 By Mitra S.K ADMIN
![Estimating Cobalt by UV-Vis Spectroscopy: The [CoCl?]²? Acetone Method](https://mitrask.com/uploads/blogs/1764834098Estimating%20Cobalt.png)
Posted on December 04 2025 By Mitra S.K ADMIN
Posted on December 04 2025 By Mitra S.K ADMIN

Posted on November 12 2025 By Mitra S.K ADMIN

Posted on September 23 2025 By Mitra S.K ADMIN

Posted on August 01 2025 By Mitra S.K ADMIN

Posted on July 25 2025 By Mitra S.K ADMIN

Posted on July 18 2025 By Mitra S.K ADMIN

Posted on July 01 2025 By Mitra S.K ADMIN

Posted on May 22 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on January 24 2025 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN
Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 31 2024 By Mitra S.K ADMIN

Posted on December 03 2024 By Mitra S.K ADMIN

Posted on October 17 2024 By Mitra S.K ADMIN

Posted on October 04 2024 By Mitra S.K ADMIN

Posted on September 13 2024 By Mitra S.K ADMIN

Posted on August 27 2024 By Mitra S.K ADMIN

Posted on August 23 2024 By Mitra S.K ADMIN

Posted on June 27 2024 By Mitra S.K ADMIN

Posted on June 22 2024 By Mitra S.K ADMIN

Posted on June 15 2024 By Mitra S.K ADMIN

Posted on May 24 2024 By Mitra S.K ADMIN
Posted on May 17 2024 By Mitra S.K ADMIN

Posted on May 09 2024 By Mitra S.K ADMIN

Posted on April 20 2024 By Mitra S.K ADMIN

Posted on April 13 2024 By Mitra S.K ADMIN

Posted on April 30 2024 By Mitra S.K ADMIN

Posted on April 29 2024 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 30 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 27 2023 By Mitra S.K ADMIN

Posted on December 26 2023 By Mitra S.K ADMIN

Posted on April 05 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on April 06 2022 By Mitra S.K ADMIN

Posted on November 28 2022 By Mitra S.K ADMIN

Posted on June 14 2022 By Mitra S.K ADMIN






